aaa+ protein|early signs of amyloidosis : Manila AAA+ ATPases use energy from ATP binding and hydrolysis to move, remodel and destroy biomolecules in key cellular processes. Multimeric rings of AAA+ ATPase domains cooperate with specific . WEBNow On Air Samo hitovi najbolje narodne muzike Samo hitovi najbolje narodne muzike 13:00 24:00 Nedeljni raspored emisija radio futoga Ponedeljak Utorak Sreda Četvrtak Petak Subota Nedelja Choose a day Ponedeljak Utorak Sreda Četvrtak Petak Subota Nedelja Samo hitovi najbolje narodne muzike 00:00Ponedeljak 00:00Ponedeljak Bez obzira da li .
0 · early signs of amyloidosis
1 · amyloidosis symptoms mayo clinic
2 · amyloidosis life expectancy mayo clinic
3 · al vs aa amyloidosis
4 · aa vs al amyloid
5 · aa amyloidosis treatment guidelines
6 · aa amyloidosis life expectancy
7 · aa amyloidosis disease life expectancy
8 · More
Our terms and conditions provide you with all of the detailed i.
aaa+ protein*******AAA+ proteins are ATPases associated with various cellular activities. AAA+ proteins contain structurally conserved ATP-binding .AAA proteins have entered the molecular realm after being known primarily for their wide range of different functions. Structural studies have highlighted the organization of their constituent ATPase domains and indicate that hexamerization in combination with unfoldase activity is a common underlying feature of this ubiquitous protein family. AAA+ ATPases use energy from ATP binding and hydrolysis to move, remodel and destroy biomolecules in key cellular processes. Multimeric rings of AAA+ ATPase domains cooperate with specific .
Molecular Machines Involved in Protein Transport across Cellular Membranes. Harald W. Platta, . Ralf Erdmann, in The Enzymes, 2007. B AAA ATPASES IN PROTEIN TRANSPORT 1 ER‐Associated Protein Degradation. The AAA‐type ATPase Cdc48p/p97 is perhaps the best‐studied AAA protein.Misfolded secretory proteins are exported from .
The mitochondrial membrane-bound AAA protein Bcs1 translocate substrates across the mitochondrial inner membrane without previous unfolding. One substrate of Bcs1 is the iron–sulfur protein (ISP .AAA proteins have an N‐terminal non‐ATPase domain that is followed by either one or two AAA domains (D1 and D2). In some proteins with two AAA domains, both are evolutionarily well conserved (like in Cdc48p/97). In others, either the D2 domain (like in Pex1p and Pex6p) or the D1 domain (in Sec18p/NSF) is better conserved in evolution (Figure 21.2) . 2. Secondary and Tertiary Structural Features Defining the AAA+ Protein Fold. The AAA+ family is a subset of the larger P-loop protein superfamily [].All P-loop family members are structurally similar and possess a distinct α/β fold. The AAA+ domain contains 200–250 amino acids with a central β-sheet in β5-β1-β4-β3-β2 strand order .
In many cases, AAA+ proteins form a ring structure that translocates a polymeric substrate through the central channel using specialized loops that project into the central channel. We discuss the major features of AAA+ protein structure and function with an emphasis on pivotal aspects elucidated with archaeal proteins.
Powered by cycles of ATP binding and hydrolysis, conformational changes in AAA+ ATPases can generate mechanical work that unfolds a substrate protein inside the central axial channel of ATPase ring for degradation. Three-dimensional visualizations of several AAA+ ATPase complexes in the act of substrate processing for protein . m-AAA proteases. The m-AAA proteases are embedded in the inner membrane with their active site oriented towards the matrix. There are two different versions of m-AAA; one is composed entirely of the homoligomers of the Afg3l2 protein; the other is a hetero-oligomeric complex of the Afg3l2 and Paraplegin proteins.
Furthermore, dysregulation of AAA protein activity has been linked to human disease, and these enzymes have emerged as potential targets for pharmacological modulation, especially in cancer 8,9 . The AAA protein spastin is needed for cell division and organelle transport. Testing spastin constructs with engineered mutations resulted in the identification of a chemical probe to analyze .
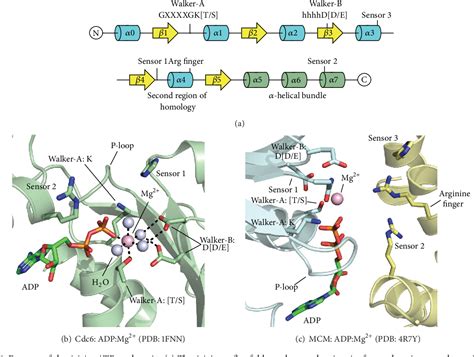
To maintain protein homeostasis, AAA+ proteolytic machines degrade damaged and unneeded proteins in bacteria, archaea and eukaryotes. . Gerdes, F., Tatsuta, T. & Langer, T. Mitochondrial AAA . Structure of the AAA+ module. (a) Monomeric AAA+ module of Aquifex aeolicus DnaA, a protein involved in the initiation of DNA replication (Protein Data Bank (PDB) code 2HCB) [5]. The α-helices and random coils are in green and the β-strands of the core αβα nucleotide-binding domain are in blue, with the exception of the two equal . The AAA protein Msp1 extracts mislocalized tail-anchored membrane proteins and targets them for degradation, thus maintaining proper cell organization. How Msp1 selects its substrates and firmly engages them during the energetically unfavorable extraction process remains a mystery. To address this question, we solved cryo-EM .early signs of amyloidosis The AAA protein Msp1 extracts mislocalized tail-anchored membrane proteins and targets them for degradation, thus maintaining proper cell organization. How Msp1 selects its substrates and firmly engages them during the energetically unfavorable extraction process remains a mystery. To address this question, we solved cryo-EM . The AAA protein spastin is needed for cell division and organelle transport. Testing spastin constructs with engineered mutations resulted in the identification of a chemical probe to analyze .

To maintain protein homeostasis, AAA+ proteolytic machines degrade damaged and unneeded proteins in bacteria, archaea and eukaryotes. . Gerdes, F., Tatsuta, T. & Langer, T. Mitochondrial AAA .aaa+ protein early signs of amyloidosis To maintain protein homeostasis, AAA+ proteolytic machines degrade damaged and unneeded proteins in bacteria, archaea and eukaryotes. . Gerdes, F., Tatsuta, T. & Langer, T. Mitochondrial AAA . Structure of the AAA+ module. (a) Monomeric AAA+ module of Aquifex aeolicus DnaA, a protein involved in the initiation of DNA replication (Protein Data Bank (PDB) code 2HCB) [5]. The α-helices and random coils are in green and the β-strands of the core αβα nucleotide-binding domain are in blue, with the exception of the two equal . A TPases a ssociated with diverse cellular a ctivities (AAA proteins) utilize the energy of ATP hydrolysis to facilitate numerous functions in the cell, such as degrading proteins (Pickart and Cohen, 2004), dissolving protein aggregates (Sanchez and Lindquist, 1990), or moving proteins across membranes (Ye et al., 2001; Gardner et al., .AAA + protein might work as a chaperone-like ATPase associated with the assembly and disassembly of protein complexes. ABC transporters, present at membranes, act as pumps, as regulators of other membrane proteins, and as channels. The contention described here is more speculative than likely, but accumulation of knowledge on . AAA proteins in mitochondria are involved in crucial processes, such as organelle fusion, protein quality control, mitochondrial protein translation and degradation 16. Abstract. Mdn1 is an essential AAA (ATPase associated with various activities) protein that removes assembly factors from distinct precursors of the ribosomal 60S subunit. However, Mdn1's large size (∼5,000 amino acid [aa]) and its limited homology to other well-studied proteins have restricted our understanding of its remodeling function.Summary. Mdn1 is an essential AAA (ATPase associated with various activities) protein that removes assembly factors from distinct precursors of the ribosomal 60S subunit. However, Mdn1’s large size (∼5,000 amino acid [aa]) and its limited homology to other well-studied proteins have restricted our understanding of its remodeling function.
cd00009 (PSSM ID: 99707): Conserved Protein Domain Family AAA, The AAA+ (ATPases Associated with a wide variety of cellular Activities) superfamily represents an ancient group of ATPases belonging to the ASCE (for additional strand, catalytic E) division of the P-loop NTPase fold
AAA proteins participate in cellular processes that include protein degradation, vesicle-mediated protein transport, cell-cycle regulation, organelle biogenesis and gene expression 17,18.In cases where re-activation of the target AAA protein function may be desirable over irreversible inhibition, for example, when a AAA protein’s functions in different processes are separated in time, reversible covalent inhibitors could be obtained using alpha cyano-substituted acrylamides 34. As the targeted engineered cysteine is located .
30/07/2022. IMAGENS FORTES! Briga de trânsito termina e.
aaa+ protein|early signs of amyloidosis